What We Do
We help executives stay ahead of quality and compliance issues in addition to leading and supporting remediation activities. Our areas of expertise include: Quality Management Systems (QMS) Gap Assessment, Remote Audits, and Compliance Remediation. We also help resolve capacity and capability gaps in these areas, all while offering the best value for our client’s money in the industry.
In addition, we offer technology (Quality 4.0) solutions focused on quality assurance and control processes. More information can be found on our Technology Solutions page.
How We Do It
QMS Gap Assessment
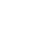
We identify common issues, gaps and opportunities for improvement across multiple regulatory and operational dimensions. Leveraging advanced analytics, we provide teams with actionable insights to streamline activities and improve operations and performance.
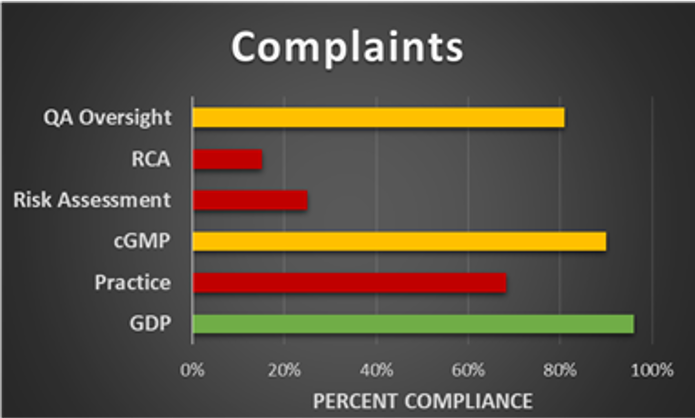
Video-Enabled Remote Audits
Our 360° camera and video conference technology allows companies to conduct effective and efficient remote audits, enabling cost effective and multi-functional team collaboration. In addition to our technology, we can supplement audit teams with experienced consultants.
Compliance Remediation
When it comes to compliance remediation, our expertise includes; Response Development, FDA Interaction Support, Third Party Assessments, Document Reviews, Remediation Planning and Execution, Deep Dive on QS Improvement Opportunities, QS Simplification and Harmonization, and Training and Mentoring.
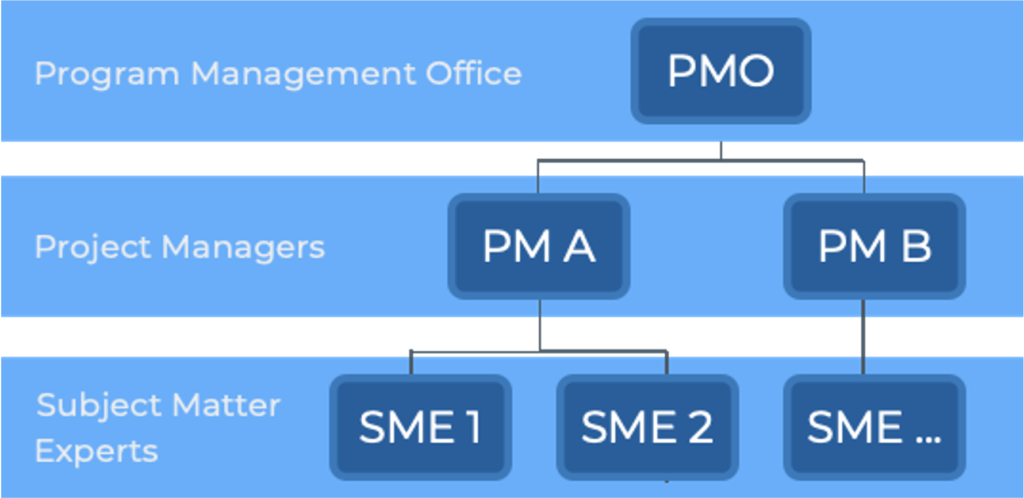
Capacity & Capability Gaps
Our consultants play roles that include Quality Engineers, Deviation Investigators, CAPA SMEs, Change Control SMEs, Complaint Investigators, Method Validation SMEs, Sterility Assurance SMEs, and Technical Writers.