-
-
-
-
-
-

Stop Throwing Away Valuable Data From Quality Management System Document Reviews and Start Using It
-

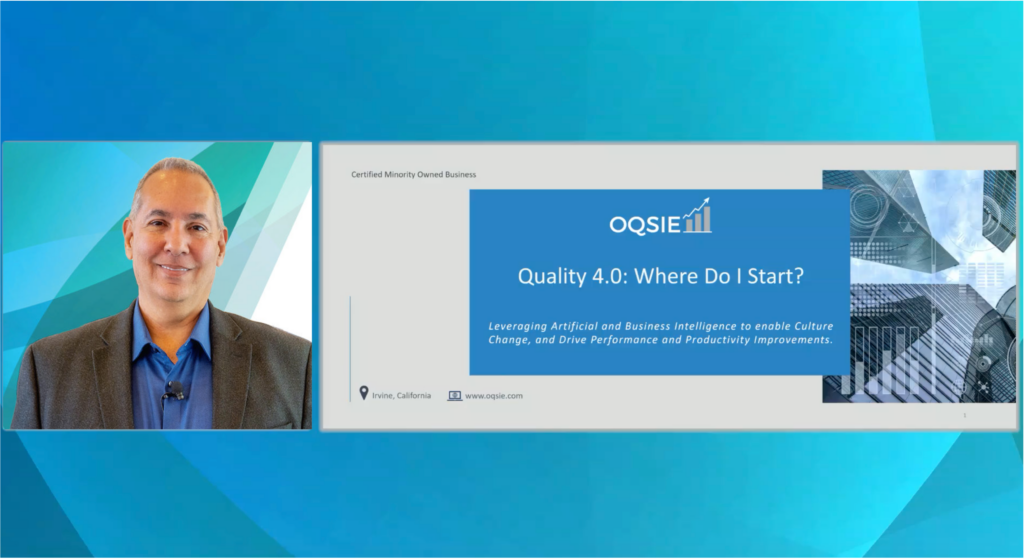
PMWS22 Workshop: Quality 4.0: Making the Shift to Continuously Monitor the Health of your QMS
-
-
-

PMWS21 Workshop: Leveraging Predictive and Behavioral Analytics to Ensure Supply Chain Health and Assess Compliance Risks
Request More Information
Please fill out the form and our team will reach out to you.